The exploration of novel biologic compounds holds significant promise for advancing medical treatments across a range of conditions. Recent research efforts have focused on investigating the therapeutic potential of one such compound, through rigorous testing in animal models.
In studies spanning various animal species and utilizing different administration methods, this compound has demonstrated broad potential across multiple tissues and conditions. In models of traumatic brain injury (TBI), the compound exhibited promising results, showing improvements in gross anatomical changes, reductions in B-amyloid protein expression, and enhancements in spatial learning and memory. These findings suggest a potential role for this compound in mitigating the effects of brain trauma and improving cognitive function.
Additionally, the compound has shown efficacy in models of melanoma, with reductions in tumor size, prevention of new tumor formation, and decreased invasiveness biomarkers observed in melanoma mouse xenografts. Similarly, in models of skin wrinkling induced by squalene monohydroperoxide, the compound demonstrated enhanced reversal of skin wrinkling, hinting at its potential as an anti-aging treatment for skin.
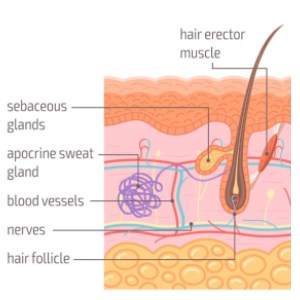
Moreover, the compound has shown promise in addressing hair loss (alopecia), with evidence of hair re-growth observed in experimental models. In gerontological studies, chronic administration of the compound resulted in increased lifespan in both mice and fruit flies, highlighting its potential as an anti-aging intervention.
Importantly, observations from these studies suggest a favorable safety profile for the compound, with no adverse effects noted in animals subjected to prolonged administration. While formal safety studies are needed to confirm these findings, the absence of growths, abscesses, infections, immune reactions, or behavioral changes is promising.
In summary, the research conducted on this compound in animal models offers valuable insights into its potential therapeutic applications across various medical conditions. Further studies are warranted to explore its efficacy and safety in human subjects, with the ultimate goal of translating these findings into tangible clinical benefits for patients.
